Researchers develop lithium-sulfur battery that can be cut, folded
A group led by scientists from the University of Electronic Science and Technology of China has created a lithium-sulfur (Li-S) battery that reportedly offers exceptional stability and safety capabilities.
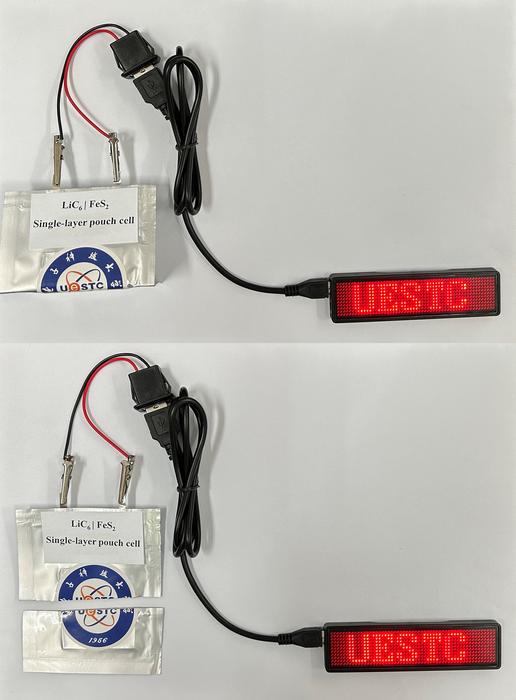
One of the fabricated battery pouch cells was even able to work after being folded and cut off. “That proves its high safety for practical application,” the researchers emphasized.
Conversion-type transition-metal sulfides (MSx) are promising cathodes for lithium-ion batteries (LIBs) due to their low cost, easy availability, and high energy density. However, MSx faces cycle stability issues in high temperatures due to polysulfide shuttling, substantial volume expansion, and sluggish reaction kinetics. To solve this, the group had previously proposed using a carbonate-based electrolyte to separate the two electrodes, an iron sulfide cathode and a lithium metal-containing anode. This solution, however, created a new problem.
“The incompatibility of MSx with a carbonate-based electrolyte is a critical challenge of this battery chemistry,” they said. “More specifically, the side reactions of polysulfides with carbonate solvents form passivating precipitates on the electrode surface, creating a significant kinetic barrier for subsequent charge transfer steps. This eventually leads to an electrode blockage and battery failure.”
To solve this issue, the group coated the iron sulfide cathodes with different polymers, which reduced corrosion without reducing functionality and rechargeability. Specifically, they used polyacrylic acid (PAA), polyacrylamide (PAM), and polyethylene oxide (PEO), as they all contain functional groups with a chelation effect. “The chelation effect is based on the coordination of lone pair electrons on oxygen or nitrogen and empty orbitals of multivalent transition metals,” the academics explained.
Through electrochemical performance tests, the scientists found that PAA performed best, retaining the electrode’s discharge capacity after 300 charge-discharge cycles. They then incorporated a PAA-coated iron sulfide (FeS2) cathode into a prototype battery design comprising a carbonate-based electrolyte, a lithium metal foil as an ion source, and a lithium carbide (LiC6) anode.
The testing also showed that the LiC6||FeS2 pouch cell exhibited no capacity degradation over 100 cycles and excellent safety when folded or cut in two. Satisfactory stability was also achieved over 300 cycles, with 72.0% capacity retention. “The FeS2 cathode, empowered by chelating-type binders, can work reversibly in the carbonate-based electrolyte at a high specific capacity of approximately 527.3 mAh g−1 for 300 cycles, even with 76.9% retention under a high loading of 5.6 mAh cm−2,” they added.
The group also applied the polymer coating to cathodes made from other metals, namely molybdenum (MoS6) and vanadium (VS4), creating LiC6||MoS6 and LiC6||VS4 structures, respectively. “LiC6||MoS6 full cells demonstrated excellent cycling stability for 300 cycles with the same high capacity as the half-cell (Li||MoS6), and the same situation also occurred in LiC6||VS4,” they stated.
Their findings were presented in “Chelating-Type Binders toward Stable Cycling and High-Safety Transition-Metal Sulfide-Based Lithium Batteries,” published in ACS Energy Letters. The team includes researchers from the University of Electronic Science and Technology of China, scientists from China’s Tianmu Lake Institute of Advanced Energy Storage Technologies, the Chinese Academy of Sciences, and Canada’s University of British Columbia.